What differentiates CVnCoV from the two mRNA vaccines from Pfizer and Moderna
The RNA to be injected has been developed with natural nucleotides (which form the bases of the genetic material) and no from artificial ones

German pharmaceutical company CureVac has developed the CVnCoV vaccine candidate formulation, which is currently in two clinical trials in phases 2/3 and 3, to evaluate its efficacy, security and immunogenicity. The company announced on April 19 that they had initiated the procedures for seeking approval. It is an injection of messenger RNA that carries the “instructions” to make the Spike (S) protein, a technology similar to both Pfizer/BioNTech and Moderna. However, unlike its competitors, the RNA to be injected has been developed with natural nucleotides (which form the bases of the genetic material) and no from artificial ones as the other two vaccines made with this technology. Like Pfizer and Moderna vaccines, CVnCoV is tested with a cell line, in this case with tumor origin and not embryonic as the other two.
mRNA is a very long chain formed by four different nucleotides whose order changes, like for SARS-CoV-2. As a numeric lock with thousands of padlock wheels, but each of them with only four options: A, C, G, and U (T for DNA), where each letter represents a different nucleotide. Both the Pfizer and Moderna vaccines use modified RNA nucleotides: one of them, uracil, U, is changed by an artificial nucleotide, to make the molecule more stable, which is very fragile by itself.
Changes in the ratio
The CureVac vaccine only uses natural nucleotides, which form the RNA that will be enveloped by lipid capsules to protect its way towards the cells. Instead of changing the nature of the molecule (they are natural instead of artificial), the German pharmaceutical company alters its ratio, achieving a higher stability. “They increase the percentages of C and G [two of the chain nucleotides] to enhance the cell’s innate immune response”, Sonia Zúñiga, a virologist from National Center for Biotechnology (CNB-CSIC, in Spanish)”, explains to Verificat. “This favours the whole immune response cascade that follows”, that is, easies the creation of antibodies and T cells, what we know as adaptive immunity. In short, the immune response is expected to be stronger than the one of Pfizer y Moderna.
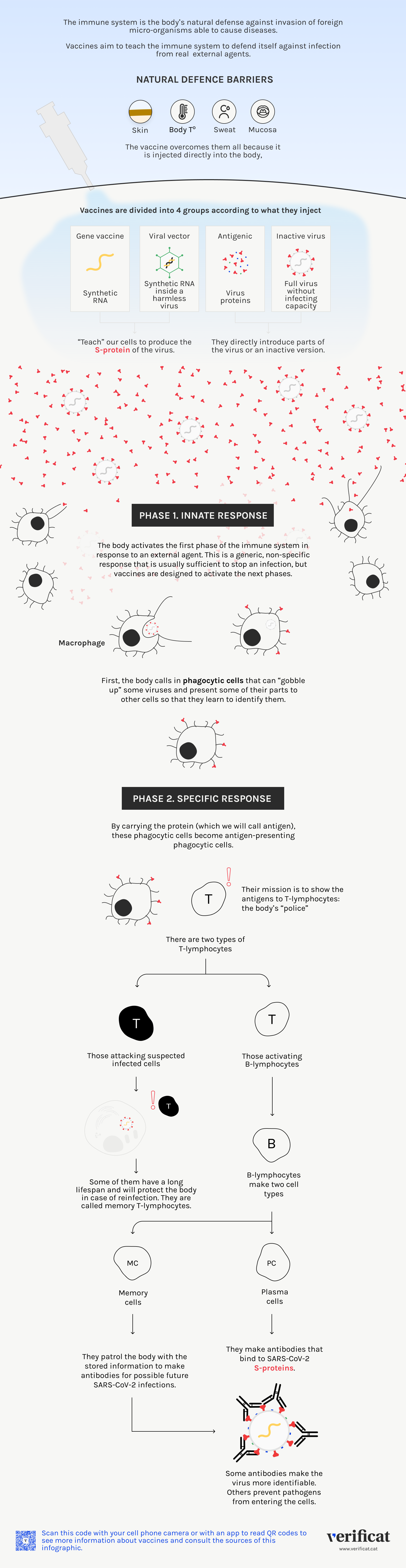
That is, the injection of Curevac allows awakening a strong immune response with a lower dose. “In the clinical trials [the scientists] use a much lower dose” than the other mRNA vaccines, Zúñiga indicates. They only inject 12 micrograms instead of the 30 used by Pfizer and 100 of Moderna. With the same amount of material, they can make more vaccines.
Stable at relatively high temperatures
One of the most outstanding features CVnCoV vaccine is that it can be kept stable at 5ºC of temperature during three months, so that it can be stored in a conventional fridge, as a difference for Pfizer’s and Moderna’s formulations, that must be kept at -70 and -20ºC, respectively. “The lipid layer that envelops it”, obtained by Curevac from a Canadian company, “makes it able to maintain three months at this temperature”, as the virologist at CNB asserts. With this, the German candidate represents a distribution opportunity in developing countries, which can lack this infrastructure to keep the injections at low temperatures.
The HeLa cell line
For its development, tests with HeLa cell lines, with tumor origin, have been developed. This is the oldest human cell line and the most used, created in 1951 from tumor cells from a patient who died from cervical cancer. It has also been tested with proteins and laboratory mice, before administering it to humans.
“Each cell line has its advantages and disadvantages, and depending on the needs one or another will be used”, Zúñiga explains. In any case, all of them “are established in the laboratory”, and if we looked at their genetic composition they would have little to do with the original, she recalls.